Study Background: Survival outcomes of older patients with AML are dismal with a 3-year survival of < 10%. The combination of hypomethylating agents (HMA) with venetoclax has emerged as the new standard of care for older unfit patients with AML, but median survival remains less than 15 months. Patients with undetectable MRD after this regimen seem to have the best long-term clinical outcomes. In this randomized phase 2 trial, the goal with combining pembrolizumab is to facilitate a more effective CTL mediated destruction of leukemic blasts resulting in improved rates of CR without MRD, which would hopefully increase duration of response and lower relapse rates. Here we report on the design of the first randomized multi-center clinical trial of HMA+venetoclax +/- ICPI in unfit AML patients.
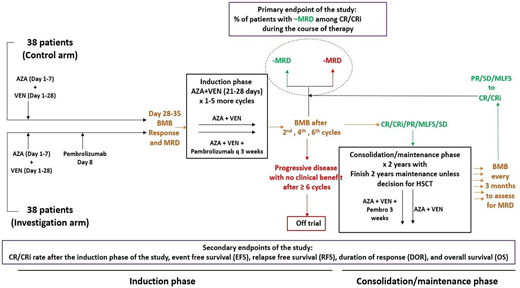
Methods: The primary objective of this Cancer Therapy Evaluation Program-approved multi-institutional, randomized phase II study (NCT04284787) is to assess the percentage of patients with MRD negative CR (MRD- CR) as measured by flow cytometry with chemotherapy + pembrolizumab during the first 6 cycles and compare between the two study arms (See study Schema). Secondary objectives include rates of CR / complete remission with incomplete count recovery (CRi), complete remission with partial recovery count (CRh) and Hematologic improvement (HI) to red blood cells and platelets, MRD negativity at Day 14, MRD-negative CR at end of induction therapy and MRD negative CR after last consolidation cycle, event free survival (EFS), relapse free survival (RFS), duration of response (DOR), and overall survival (OS), and proportion of patients who develop severe toxicity. Important exploratory objectives include MRD assessment by duplex sequencing (DS), and comparing DS and multiparameter flow cytometry for MRD detection.
A total of 76 patients will be included (38 patients in the intervention arm and 38 patients in the control arm). Planned stratification factors include 1) cytogenetics (intermediate/unknown vs. adverse karyotype) 2) antecedent hematologic disorder defined as prior MDS, MPN, or aplastic anemia (present vs. absent); and 3) reason to not receive intensive chemotherapy (ineligibility vs refusal). All patients will receive azacitidine at 75 mg/m2 IV over 10-40 minutes or SQ x 7 days on Days 1-7 or Days 5-2 (to avoid weekend administration) and, including a ramp-up phase to 400 mg /day venetoclax on Days 1-4. Venetoclax will be administered at 400 mg/day on the remaining days of the cycle for the first cycle and on Days 1-21 or 1-28 for subsequent cycles (depending on count recovery).
Half of the patients will receive pembrolizumab intravenously starting on Day 8 of the first cycle and then q 3 weeks in cycles 2-6 (intervention arm), up to 2 years of maintenance. The other half will not receive pembrolizumab (control arm). After 15 efficacy-evaluable patients in each of the two study arms have had their primary endpoint results available, a formal futility and toxicity analysis will be conducted. Follow up will continue up to 2 years after the last patient is randomized
Eligible patients are aged ≥60 years with newly diagnosed and pathologically-confirmed AML, including secondary AML arising from prior MDS and therapy-related AML, who are deemed ineligible for intensive chemotherapy according to treating physician's assessment or who refuse intensive chemotherapy. Candidates with high risk MDS and secondary AML arising from myeloproliferative neoplasm or other hematologic disorders are not allowed. Additional exclusion criteria include CBF-AML, prior allogeneic stem cell transplant, receipt of prior hypomethylating therapy for antecedent MDS, active infection requiring systemic therapy, and use of corticosteroids.
Responses in AML patients will be assessed using European Leukemia Net 2017 response criteria. EFS will be calculated as the time from initial treatment to either disease relapse or death. OS will be calculated from time from initial treatment to death. Biomarker analyses will include an extensive array of immunologic and correlative studies that will evaluate PD-1 and PD-L1 expression, immune cell subset analysis, leukemia specific T-cell response, cytokine level, T-cell receptor (TCR) repertoire, as well as RNA-seq, gut microbiome characterization and metabolomics, and whole exome sequencing for tumor and germline DNA.
Zeidan:Abbvie: Consultancy, Honoraria, Research Funding; MedImmune/Astrazeneca: Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria; Celgene / BMS: Consultancy, Honoraria, Research Funding; Trovagene: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria; Boehringer-Ingelheim: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Acceleron: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; BeyondSpring: Consultancy, Honoraria; Cardiff Oncology: Consultancy, Honoraria, Other; Takeda: Consultancy, Honoraria, Research Funding; Ionis: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Leukemia and Lymphoma Society: Other; CCITLA: Other; Astex: Research Funding; Aprea: Research Funding; ADC Therapeutics: Research Funding; Taiho: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Cardinal Health: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Otsuka: Consultancy, Honoraria. Radich:Amgen: Consultancy; Bristol-Myers Squibb: Consultancy; Jazz: Consultancy; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal